Novartis Pharmaceuticals Canada Inc v Teva Canada Limited, 2015 FC 770
The law on patent promises inched forward once again after the recent Federal Court (“the Court”) decision in Novartis v Teva. During PM(NOC) proceedings, Novartis was able to uphold its Canadian Patent No. 2,255,951 (“the ‘951 patent”) against allegations of invalidity from Teva, but not without the Federal Court making a number of razor thin distinctions between what the ‘951 patent promised and what it did not. In doing so, the Court focused on the fact that the ‘951 patent did not assert that the claimed compounds had been shown to be useful for treatment in humans to ultimately reject that the patent made such a promise.
Teva’s Allegation of an Overarching Promise
Teva alleged that the ‘951 patent, which covers compounds and uses of those compounds for binding to iron in the treatment of iron overload disorders, [1] makes the overarching promise across all of its claims that certain compounds will (or have been found to) be useful in treating iron overload disorders in humans. [3]
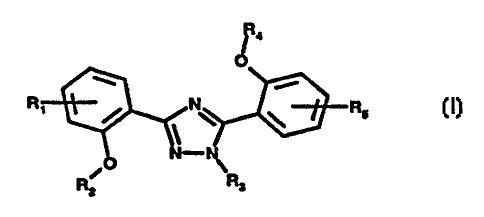
The description states that the invention relates to “the use of compounds of the formula I . . . in the treatment of diseases which cause an excess of metal in the human or animal body . . . in particular, in a method for the therapeutic treatment of the human body”. [29] Claims 1 to 4 of the ‘951 patent claim the compounds “for use in treatment of a disease…”, [30] and the description states that the compounds have valuable pharmaceutical properties when used in the treatment of such disorders:
It has now been found that certain substituted 3,5-diphenyl-1,2,4-triazoles have valuable pharmaceutical properties when used in the treatment of disorders which cause an excess of metal in the human or animal body or are caused by it, primarily a marked binding of trivalent metal ions, in particular those of iron. [15]
But the Court found no such promise. The quoted phrase, which was at the centre of the dispute, was interpreted by the Court as meaning that the compounds are valuable when used in the treatment of iron overload disorders, not that they are valuable in treating iron overload disorders. [22] The statement was read to be merely stating that a beneficial property of the compound was that it could markedly bind to iron. [24] Although binding to iron is undoubtedly important to iron overload treatment, the promise did not go so far.
The Court found that the specification, and in particular the abstract, merely states that the compounds can be used in the treatment of iron overload disorders, not that they have been found to be useful in the treatment of iron overload disorders. [27] In terms of evidence of utility, the patent only discloses in vitro studies and in vivo studies done in animals, not in humans. [28] There was no express promise of treating iron overload disorders in humans. [32]
The promise was merely that the compounds had capacity to bind to iron both in vitro and in vivo (in animal studies) and that they were able to induce iron excretion. [39] This promised utility was demonstrated or at least soundly predicted. [57] Teva’s assertion that the quoted statement made an overarching promise across all claims that the claimed compounds are useful in treating iron overload disorders was therefore rejected. [30]
The Novartis Patent Promise
Although the Court rejected the overarching promise proposed by Teva, it went on to find that claims 1 and 40-42 (the “use” claims) make a promise that could not have been demonstrated or soundly predicted. [41]
Claims 1 and 40, for example, are reproduced in part:
- A compound of formula I in which R1 and R5 simultaneously or independently of one another are hydrogen, halogen, hydroxyl, …
or a pharmaceutically acceptable salt thereof;
for use in treatment of a disease which causes an excess of metal in a human or animal body or a disease which is caused by the excess of metal in the human or animal body.
- The use of a compound according to any one of claims 5 to 37, or a pharmaceutically acceptable salt thereof in production of a pharmaceutical preparation for treatment of an excess of iron in a human or animal body. [2,255,951, emphasis added]
The Court sided with both experts’ opinion that these claims promised use of these compounds in the treatment of human diseases, but a lot of work needed to be done before an actual compound was selected for treatment. [41] Without stating much more, and despite this finding, the Court found that, overall, Teva’s allegations of inutility were unjustified. [42]
Obviousness
In the alternative, Teva argued that if the utility of the compounds is merely their capacity to bind iron, then the compounds of the invention were obvious. [18] However, given that it was not in the prior art that there is a class of compounds which are soluble in vivo and also capable of inducing iron excretion, [49] the Court thought the isolating these compounds was inventive, and that the work involved in reaching them was not obvious to try. [54-55]
Insufficiency
Teva also argued that, based on its construction of the claims, the real invention that would treat iron overload disorders in humans was buried in claim 32. [56] Having rejected this construction, Court simply affirmed that the utility of being able to bind iron and induce iron excretion was fully disclosed. [57]
In sum, since each of Teva’s allegations of invalidity was rejected, the Court prohibited the Minister of Health from issuing a Notice of Compliance to Teva. [64]
Commentary
The Court seemed to find it particularly determinative that the patent did not state that the compounds were even shown to be useful for treatment in the past. Without such statements of past successes, and without an express promise, [32] the Court could find no such promise.
It would seem that the ‘951 patent sits in the sweet spot where it is able to claim certain compounds for use in the treatment of a particular disease, without having to provide evidence that the compounds would be useful in treating such a disease – only that the compounds would be useful in fulfilling the underlying mechanism of action that will be used in the treatment of that disease.