Gilead Sciences, Inc v Canada (Health), 2015 FC 610
In Patented Medicines (Notice of Compliance) (“PM(NOC)”) proceedings between Gilead Sciences Inc. (“Gilead”) and Apotex Inc. (“Apotex”), the Federal Court dismissed Gilead’s assertion that its Canadian Patent No. 2,298,059 (“the ‘059 patent”) is valid. Making that assertion would be relitigating old issues and an abuse of PM(NOC) proceedings.
Gilead had already conceded that claims 1, 2, 5, 6, and 7 of the ‘059 patent were invalid in earlier PM(NOC) proceedings with another generic, Teva Canada Ltd. (“Teva”) (2013 FC 1270). [4] Claims 3 and 4 were also found to be obvious in those proceedings. [4] Now, in proceedings with Apotex, Gilead sought to reassert the validity of the entire patent. [8] Gilead insisted that it would fill an evidentiary gap this time around, and that claims 1, 2, 5, 6, and 7 being at issue could support a different outcome, but each of those justifications were rejected. [6, 8]
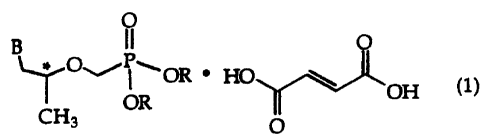
The Court reiterated that it is settled law that an attempt to relitigate the validity of a patent is an abuse of process. [9] Once the validity of a patent is put into play in PM(NOC) proceedings, the patentee must fully defend the assertion. [13] The patentee cannot concede some claims in one proceeding but later resile from that position in another. [14] The Court explained its reasoning:
“If it were otherwise, the patentee could effectively split its case and unilaterally compel subsequent generic challengers to litigate claims, the invalidity of which the patentee had effectively conceded. This would amount to a manipulation of the system and it would violate the principle that the patentee is required to put its strongest case forward in the first instance.” [14]
The Court concluded by suggesting that if Gilead is aggrieved by the earlier finding in the proceeding with Teva, then it could always bring an infringement action. [16]