- Filter by
- Categories
- Tags
- Show all
- Article
- Confusion
- Contract
- Copyright
- Corporate
- Domain Names
- Employment
- Expungement
- Expungement
- Grey Marketing
- Industrial Designs
- Licensing
- Obviousness
- Opposition
- Passing Off
- Patent
- Patent Infringement
- Patent Strategy
- PCK News
- PM(NOC)
- Procedure
- Prosecution
- Services
- Summary
- Trade Secrets
- Trademark
- Use
- Validity
- Abandonment
- Abstract Theorems
- Abuse of Process
- Accounting of Profits
- Alice/Mayo Test
- Ambiguity
- Anticipation
- Appeal
- Apple
- Artificial Intelligence
- Assignment
- Bargain Theory
- Biosimilar
- Biotech Patents
- Branding
- business
- CAFC
- Canada
- Certificate of Supplementary Protection
- Chemical Patent
- CIPO
- claim
- Claim Construction
- Class Actions
- College of Patent Agents and Trademark Agents
- combination drugs
- Commercial Success
- Common General Knowledge
- Confidential Information
- Confusion
- Contract
- Copyright
- Copyright Infringement
- Costs
- Counterfeit
- Court of Appeals for the Federal Circuit
- COVID-19
- Damages
- Data Protection
- Design Patents
- Distinctiveness
- Diversity
- DNA Patents
- Domain Names
- Dosage Range
- Double Patenting
- Due Care
- E-Commerce
- Enablement
- Estoppel
- Evidence
- Expert Evidence
- Fair Use
- Federal Court
- Federal Court of Appeal
- File Wrapper Estoppel
- Food and Drug Regulations
- Fraud
- funding
- Generic Drugs
- Hague Agreement
- Indefiniteness
- Induced Infringement
- Industrial Designs
- Injunction
- Innovation
- Innovative Drugs
- Insufficient Disclosure
- Intellectual Property
- Inter Partes Review
- Investors
- IP Litigation
- IP Strategy
- IP Treaty
- IPIC
- Jurisdiction
- Law Firm
- Licensing IP
- Madrid Protocol
- Methods of Medical Treatment
- Non-Infringing Alternative
- Non-Practicing Entity
- Novelty
- Obviousness
- Official Marks
- On-Sale Bar
- Overbreadth
- Ownership & Inventorship
- Passing Off
- Patent Act
- Patent Agent
- Patent Appeal Board
- Patent Application
- Patent Drafting
- Patent Fees
- Patent Infringement
- Patent Law
- Patent Lawyer
- Patent Litigation
- Patent Prosecution
- Patent Rules
- Patent Strategy
- Patent Term Adjustment
- Patent Textbook
- Patent Trolls
- Patent Validity
- Patentable Subject Matter
- Patents
- PCT
- Pharmaceutical Patent
- Pharmaceutical Pricing
- PM(NOC)
- PMPRB
- Prior Art
- Prior Disclosure
- prior use
- Priority
- Privilege
- Product Specificity
- Promise Doctrine
- Provisional Patent
- Punitive Damages
- Reinstatement
- Remedies
- SCOTUS
- Selection Patent
- Software
- Software Copyright
- Software Patent
- Sound Prediction
- Springboard Profits
- SRED
- Standard of Review
- Start-up
- Startups
- Supreme Court of Canada
- Technology
- Trade Secrets
- Trademark
- Trademark Agent
- Trademark Expungement
- Trademark Infringement
- Trademark Law
- Trademark Opposition
- Trademark Registration
- Trademarks Act
- United States
- Use
- USPTO
- Utility
May 1, 2019
May 1, 2019
2019 FC 406 - FC confirms that registered owner is not precluded to submit new evidence on appeal if no evidence is submitted after receiving s.45 notice from the Registrar.
April 10, 2019
April 10, 2019
2018 FC 1261 - Trademarks expunged for lack of evidence establishing use, and for using deviants without the dominant features of the trademark design.
September 20, 2018
September 20, 2018
2018 FC 689 – Federal Court confirms that material facts must be pleaded in order to succeed with defence of prior disclosure.
September 19, 2017
September 19, 2017
2016 FC 857 - The FC granted Gilead’s application for an order prohibiting the Minister of Health from issuing a Notice of Compliance to Apotex in respect of its Notice of Allegation until the expiry of Gilead’s Canadian patent.
August 22, 2017
August 22, 2017
2016 FCA 161 - The FCA remitted a proceeding back to the FC for redetermination after agreeing with Pfizer that part of Teva’s evidence in the FC decision was based on hearsay.
August 1, 2017
August 1, 2017
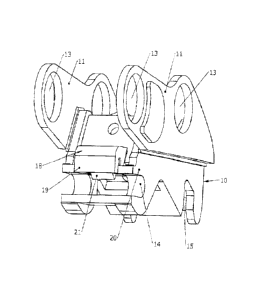
2016 FC 1117 - Cascade brought a claim that Kinshofer had infringed its Canadian patent related to a safety locking device for quick couplers. After carrying out a claim construction, the FC did not find that the patent had been infringed.
June 13, 2017
June 13, 2017
2016 FC 382 - The FC concluded that Apotex’s allegations of non-infringement in respect of Shire's Canadian Patent were justified and accordingly denied Shire’s requested prohibition order.
October 3, 2016
October 3, 2016
2015 FCA 191 - The FCA dismissed an appeal wherein Alcon sought to reverse a finding of invalidity against its patent by surreptitiously asking the FCA to reweigh the evidence as a challenge against the Federal Court Judge’s findings of fact and preferred expert evidence.
April 12, 2016
April 12, 2016
2015 FC 391 - The Federal Court recognized the increasing and improper use of the phrase "under advisement" in response to questions on examination for discovery.
March 30, 2016
March 30, 2016
2015 FCA 116 - The FCA advised that where expert evidence plays a significant role, claim construction might involve subsidiary factual disputes reviewed on a palpable and overriding error standard, which is equivalent to the United States clear error standard.
September 2, 2015
September 2, 2015
AstraZeneca Canada Inc v Apotex Inc, 2015 FC 322 - Claim 1 was worded general enough to capture Apotex’s subcoating layer even though Apotex’s subcoating layer was generated by an in situ chemical reaction, a process that the patentee had not contemplated.
July 5, 2015
July 5, 2015
Tan-Jen Ltd v Di Pede, 2015 ONSC 3685 - In a copyright infringement case regarding supposedly one-of-a-kind design moulds for a home's exterior, the Court allowed inspection of the property for valuation purposes and refused a request to keep the court proceedings closed from the public.
February 3, 2015
February 3, 2015
Moore v Getahun, 2015 ONCA 55 - The Court referred to UK authorities that described patent law as an example of a highly technical area where “expert witnesses require a high level of instruction by the lawyers”, supposedly to liken the highly technical area of patent law to the highly technical area of medical malpractice with respect to its reliance on expert evidence.
October 27, 2014
October 27, 2014
Dow Chemical Co v NOVA Chemicals Corp, 2014 FC 844 - The Federal Court found that NOVA Chemicals infringed Canadian Patent No. 2,160,705, owned by The Dow Chemical Company, by NOVA’s use of its “SURPASS” polyethylene product. Allegations of invalidity for lack of utility, claims broader than any invention made or disclosed, anticipation, obviousness, double patenting, and insufficiency of the specification were unsuccessful.
February 25, 2013
February 25, 2013
Apotex Inc. v. Eli Lilly Canada Inc., 2013 FCA 45 This was an appeal by Apotex from a FC decision upholding the order of Prothonotary Aronovitch […]
January 31, 2013
January 31, 2013
Monsanto Canada Inc. v. Verdegem, 2013 FC 50 Monsanto commenced an action for infringement of its patent covering genetic material found in plant seeds. Verdegem was […]