- Filter by
- Categories
- Tags
- Show all
- Article
- Confusion
- Contract
- Copyright
- Corporate
- Domain Names
- Employment
- Expungement
- Expungement
- Grey Marketing
- Industrial Designs
- Licensing
- Obviousness
- Opposition
- Passing Off
- Patent
- Patent Infringement
- Patent Strategy
- PCK News
- PM(NOC)
- Procedure
- Prosecution
- Services
- Summary
- Trade Secrets
- Trademark
- Use
- Validity
- Abandonment
- Abstract Theorems
- Abuse of Process
- Accounting of Profits
- Alice/Mayo Test
- Ambiguity
- Anticipation
- Appeal
- Apple
- Artificial Intelligence
- Assignment
- Bargain Theory
- Biosimilar
- Biotech Patents
- Branding
- business
- CAFC
- Canada
- Certificate of Supplementary Protection
- Chemical Patent
- CIPO
- claim
- Claim Construction
- Class Actions
- College of Patent Agents and Trademark Agents
- combination drugs
- Commercial Success
- Common General Knowledge
- Confidential Information
- Confusion
- Contract
- Copyright
- Copyright Infringement
- Costs
- Counterfeit
- Court of Appeals for the Federal Circuit
- COVID-19
- Damages
- Data Protection
- Design Patents
- Distinctiveness
- Diversity
- DNA Patents
- Domain Names
- Dosage Range
- Double Patenting
- Due Care
- E-Commerce
- Enablement
- Estoppel
- Evidence
- Expert Evidence
- Fair Use
- Federal Court
- Federal Court of Appeal
- File Wrapper Estoppel
- Food and Drug Regulations
- Fraud
- funding
- Generic Drugs
- Hague Agreement
- Indefiniteness
- Induced Infringement
- Industrial Designs
- Injunction
- Innovation
- Innovative Drugs
- Insufficient Disclosure
- Intellectual Property
- Inter Partes Review
- Investors
- IP Litigation
- IP Strategy
- IP Treaty
- IPIC
- Jurisdiction
- Law Firm
- Licensing IP
- Madrid Protocol
- Methods of Medical Treatment
- Non-Infringing Alternative
- Non-Practicing Entity
- Novelty
- Obviousness
- Official Marks
- On-Sale Bar
- Overbreadth
- Ownership & Inventorship
- Passing Off
- Patent Act
- Patent Agent
- Patent Appeal Board
- Patent Application
- Patent Drafting
- Patent Fees
- Patent Infringement
- Patent Law
- Patent Lawyer
- Patent Litigation
- Patent Prosecution
- Patent Rules
- Patent Strategy
- Patent Term Adjustment
- Patent Textbook
- Patent Trolls
- Patent Validity
- Patentable Subject Matter
- Patents
- PCT
- Pharmaceutical Patent
- Pharmaceutical Pricing
- PM(NOC)
- PMPRB
- Prior Art
- Prior Disclosure
- prior use
- Priority
- Privilege
- Product Specificity
- Promise Doctrine
- Provisional Patent
- Punitive Damages
- Reinstatement
- Remedies
- SCOTUS
- Selection Patent
- Software
- Software Copyright
- Software Patent
- Sound Prediction
- Springboard Profits
- SRED
- Standard of Review
- Start-up
- Startups
- Supreme Court of Canada
- Technology
- Trade Secrets
- Trademark
- Trademark Agent
- Trademark Expungement
- Trademark Infringement
- Trademark Law
- Trademark Opposition
- Trademark Registration
- Trademarks Act
- United States
- Use
- USPTO
- Utility
January 9, 2018
January 9, 2018
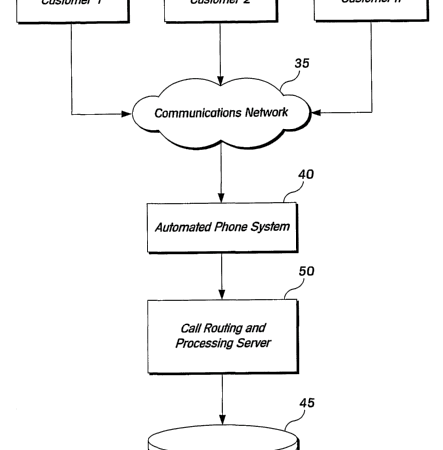
PAB 1420 - Canadian Patent Application No. 2,529,210 filed by Assurant Inc. for a system that routes customer calls based on a sales agent’s past performance was rejected by the Patent Appeal Board, at least for the reasons of non-statutory subject matter and obviousness.
January 2, 2018
January 2, 2018
2017 FCA 201 - The FCA upheld the FC’s ruling against Apotex, that Pfizer's failure to pay the proper final fee for the issuance of a Canadian patent will not invalidate the patent.
December 19, 2017
December 19, 2017
2017 FC 777 - The FC rejected Teva’s allegations that Pfizer's Canadian patent was obvious and lacked utility. The FC found that the POSITA would not have been able to predict the novel crystalline form taught by the patent, and that the subject-matter of the invention claimed in the patent was useful.
December 12, 2017
December 12, 2017
2017 FC 774 - The FC granted Pfizer's order pursuant to Section 6 of the PM(NOC) Regulations, prohibiting the Minister of Health from issuing a NOC to Apotex, with respect to a Canadian patent The FC found, on a balance of probabilities, that Apotex’s allegations of obviousness, inutility, non-infringement, overpromising, anticipation and double patenting were not justified.
December 5, 2017
December 5, 2017
2017 FC 826 - The FC declined to approve, or disapprove, Seedlings' litigation funding agreement with Bentham, where Bentham would fund Seedlings' patent litigation against Pfizer. The FC found that it lacked jurisdiction, as contractual matters are generally provincial in nature, and that only agreements related to class action proceedings would require the approval of the FC.
November 28, 2017
November 28, 2017
CIRA 344 - CIRA granted Virox its request to have the domain virox.ca transferred from Nameshield to itself, based on the domain being confusingly similar to Virox’s trademarks, Nameshield having registered the domain in bad faith and Nameshield having no legitimate interest in the domain.
November 21, 2017
November 21, 2017
2017 FCA 215 - The FCA found that trademarks owned by Travelway were confusingly similar to, and passing off on, trademarks owned by Wenger. The FCA's finding reversed a prior decision of the FC.
November 14, 2017
November 14, 2017
2016 FC 1362 - The formulation patent for the insomnia-treating drug zolpidem was found to be substantially valid, but not infringed by Pharmascience's generic version of zolpidem.
November 7, 2017
November 7, 2017
2016 FCA 267 - Apotex unsuccessfully sought to show that the FCA had erred in another decision by not following the SCC's decision in Whirlpool. Apotex also unsuccessfully argued that the FC had erred by finding the tadalafil patent to have sufficient disclosure.
October 31, 2017
October 31, 2017
2016 FC 716 - The AG was successful in bringing a motion to strike Alexion's constitutional challenge to the patented medicines price regulation scheme in the Patent Act. The motion was brought on the ground that the application was bereft of any chance in light of a line of jurisprudence, which had fully and finally determined that these sanctions are intra vires and constitutional.
October 24, 2017
October 24, 2017
2017 FC 548 - In this application for judicial review over s. 5 of the PM(NOC) Regulations, the FC agreed with the AG who argued that since another innovator also had patents listed on the Patent Register pertaining to products to which Innovator Company made comparisons, the other innovator was a necessary respondent to the application.
October 17, 2017
October 17, 2017
2016 U.S. App. LEXIS 8699 (Fed. Cir. 2016) - The US CAFC reversed the decision of a district court in part, finding that the claims in a software patent were patent-eligible, and reversed the finding that the claims were anticipated, but affirmed the district court’s decision that there was no infringement.
October 10, 2017
October 10, 2017
2017 FCA 161 - The FCA dismissed Idenix's appeal to a FC decision in which Idenix's Canadian patent was found invalid for insufficient disclosure and its counterclaim against Gilead was dismissed.
October 3, 2017
October 3, 2017
2016 FC 986 - The FC dismissed Supertek's claim that Mishan engaged in conduct contrary to Section 7(a) of the Trade-marks Act, in relation to a Canadian patent.
September 26, 2017
September 26, 2017
2017 SCC 33 - The SCC allowed Douez’s appeal and found strong reasons not to enforce the forum selection clause present in Facebook Inc.’s terms of use on its social media website, Facebook.com.
September 19, 2017
September 19, 2017
2016 FC 857 - The FC granted Gilead’s application for an order prohibiting the Minister of Health from issuing a Notice of Compliance to Apotex in respect of its Notice of Allegation until the expiry of Gilead’s Canadian patent.
September 12, 2017
September 12, 2017
2017 FC 402 - The FC dismissed the University of Alberta’s application for judicial review of three CIPO decisions, effectively refusing to declare the patent application in question in good standing following the failure of the original applicants to reply to a requisition and to apply to reinstate the application within the 12-month grace period.
September 5, 2017
September 5, 2017
2016 FC 229 - The FC heard and granted an uncontested application by Novartis, pursuant to sections 31(3) and 52 of the Patent Act, to vary all entries in the records of the Patent Office with regards to the inventorship of a Canadian Patent.
August 29, 2017
August 29, 2017
2017 FC 637 - The FC addressed three outstanding issues in the calculation of damages and profits and ordered Nova to pay Dow over $644 million for infringing Dow's Canadian patent.
August 22, 2017
August 22, 2017
2016 FCA 161 - The FCA remitted a proceeding back to the FC for redetermination after agreeing with Pfizer that part of Teva’s evidence in the FC decision was based on hearsay.